The Revolutionary Potential of CRISPR Gene Editing and Gene Therapy
What is CRISPR?
CRISPR, which stands for Clustered Regularly Interspaced Short Palindromic Repeats, is a revolutionary gene-editing technology that allows scientists to make precise changes to an organism's DNA. It was originally discovered as part of the bacterial immune system, where it helps bacteria defend against viral infections.
Biologists coined the term "CRISPR" to characterize a genetic phenomenon observed in microbes, notably bacteria and archaea, as far back as 1987. Its function became clearer around 2005 when researchers identified CRISPR as part of the microbial immune system. Microbes employ CRISPR to safeguard against viral intruders by recognizing and neutralizing specific invaders.
Emmanuelle Charpentier and Jennifer A. Doudna received the 2020 Nobel Prize in Chemistry for discovering one of gene technology's sharpest tools: the CRISPR/Cas9 genetic scissors.
What is Gene/Genome Editing and How is it related to CRISPR?
Gene editing refers to the process of making precise modifications to an organism's DNA. These modifications can involve altering, adding, or removing specific sequences of nucleotides, the building blocks of DNA, in order to change the genetic information carried by the organism.
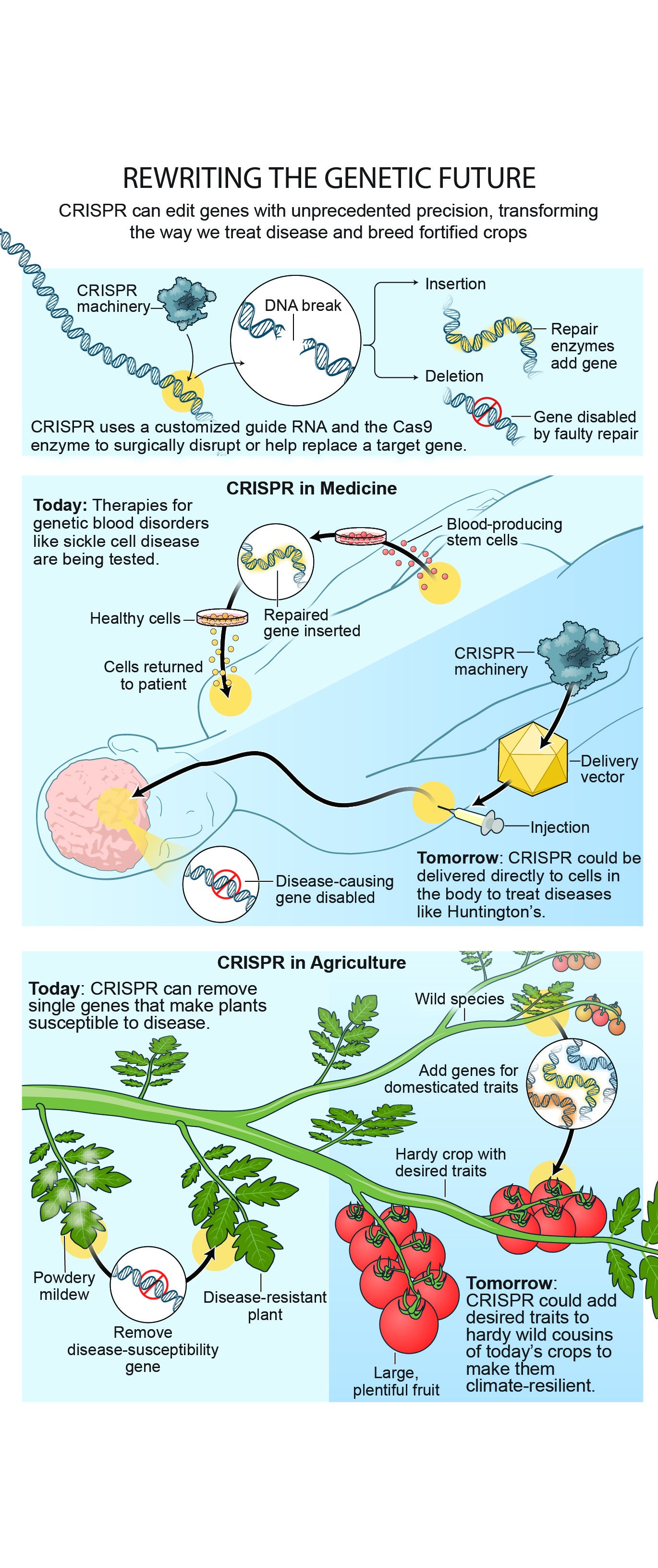
CRISPR, or Clustered Regularly Interspaced Short Palindromic Repeats, is a powerful and precise tool for gene editing. It serves as the foundation for a technique that allows scientists to make targeted changes to the DNA of organisms with unprecedented accuracy and efficiency.
CRISPR-based gene editing works by using a combination of a protein called Cas9 (or a similar enzyme) and a guide RNA (gRNA).
Cas9: a CRISPR-associated (Cas) endonuclease, or enzyme, that acts as “molecular scissors” to cut DNA at a location specified by a guide RNA
Guide RNA (gRNA): a type of ribonucleic acid (RNA) molecule that binds to Cas9 and specifies, based on the sequence of the gRNA, the location at which Cas9 will cut DNA.
How does CRISPR work?
The guide RNA is designed to match a specific sequence of DNA within the organism's genome. When introduced into cells, the Cas9 protein, guided by the gRNA, can then precisely cut the DNA at the targeted location. This cut triggers the cell's natural repair mechanisms, which can be harnessed to introduce desired genetic changes.
CRISPR-Cas9 was adapted from a naturally occurring genome editing system that bacteria use as an immune defense. When infected with viruses, bacteria capture small pieces of the viruses' DNA and insert them into their own DNA in a particular pattern to create segments known as CRISPR arrays. The CRISPR arrays allow the bacteria to "remember" the viruses (or closely related ones). If the viruses attack again, the bacteria produce RNA segments from the CRISPR arrays that recognize and attach to specific regions of the viruses' DNA. The bacteria then use Cas9 or a similar enzyme to cut the DNA apart, which disables the virus.
Researchers adapted this immune defense system to edit DNA. They create a small piece of RNA with a short "guide" sequence that attaches (binds) to a specific target sequence in a cell's DNA, much like the RNA segments bacteria produce from the CRISPR array. This guide RNA also attaches to the Cas9 enzyme. When introduced into cells, the guide RNA recognizes the intended DNA sequence, and the Cas9 enzyme cuts the DNA at the targeted location, mirroring the process in bacteria. Although Cas9 is the enzyme that is used most often, other enzymes (for example Cpf1) can also be used. Once the DNA is cut, researchers use the cell's own DNA repair machinery to add or delete pieces of genetic material, or to make changes to the DNA by replacing an existing segment with a customized DNA sequence.
How is CRISPR different from other gene-editing tools ?
According to bioengineer Stanley Qi, "Before CRISPR, most gene-editing tools were a single protein. By changing the peptide sequence of these proteins, scientists could alter their targets. To change the target, you need to completely redesign the protein’s sequence and then test if it even works, which is tedious, unpredictable, and time-consuming. These gene-editing tools were theoretically interesting, but they were difficult to use for large-scale studies and therapeutics.
Compared to that, CRISPR is elegant because the target recognition sequence is mostly encoded within an RNA rather than a protein, and redesigning this sequence is one of the simplest things you can do in molecular biology. It makes genome editing similar to operating a GPS: If you want to go to destination A, you just type the address, and to change to destination B, you just enter the new location. So, this tool dramatically reduces the burdens, cost, timing, while increasing the precision and accuracy of a gene-editing system."
How is CRISPR useful to human health and medicine?
CRISPR holds enormous potential for treating human diseases, offering a revolutionary approach to correcting genetic defects at the molecular level. While CRISPR-based therapies for human diseases are still in early stages of development, significant progress has been made in preclinical studies and clinical trials.
CRISPR could potentially correct genetic mutations responsible for inherited disorders such as cystic fibrosis, sickle cell disease, Duchenne muscular dystrophy, and Huntington's disease. By editing the defective genes, CRISPR offers the possibility of providing long-term therapeutic benefits to patients.
Conventional treatments such as small molecule drugs, surgery, and other methods often fall short in addressing certain diseases. However, CRISPR molecules have emerged as promising therapies due to their ability to precisely edit DNA within the human body. This offers not just symptomatic relief but also the potential for a permanent cure. The recent FDA approval of the inaugural CRISPR drug, Casgevy, for treating sickle cell anemia and beta thalassemia underscores both its safety and its potential for treating a range of other diseases. Sickle cell anemia, characterized by a mutation in red blood cells, traditionally necessitates frequent blood transfusions or costly and health-compromising bone marrow transplants from compatible donors. CRISPR offers a revolutionary approach by enabling a one-time treatment to correct the underlying genetic mutation permanently. This breakthrough could potentially benefit over 8,000 genetic diseases with similar prospects for treatment.
India conducted the first human clinical trial of gene therapy for haemophilia A (FVIII deficiency) at Christian Medical College (CMC) Vellore. The trials involved deploying a novel technology of using a lentiviral vector to express a FVIII transgene in the patient’s own haematopoietic stem cell which will then express FVIII from specific differentiated blood cells. This shows that gene therapy is already in the works but this trial was not related the CRISPR technology. While CRISPR/Cas9 is a promising gene-editing tool to correct mutations that underlie hemophilia and other genetic diseases, its therapeutic use still poses challenges. One particular issue is the possibility of off-target effects — unintended gene edits that may occur in other parts of the genome, potentially leading to long-term complications, including cancer.
In a news from Philadelphia, CRISPR-Cas9 gene editing was found to be safe and largely effective in addressing a form of inherited blindness in a group of patients that, for the first time, included children. In a multi-site clinical trial called BRILLIANCE that included researchers from the Perelman School of Medicine at the University of Pennsylvania and Children’s Hospital of Philadelphia (CHOP), 14 people—including two children under 17 years old—with Leber Congenital Amaurosis (LCA), a form of blindness resulting from mutations in the CEP290 gene, received a single, surgical injection of a gene editing agent. Of those 14, nearly half reported measurable improvements in sight, including the two children according to the study, published today in the New England Journal of Medicine (NEJM).
Our current knowledge of gene therapy is rapidly evolving, and the main challenge lies in ensuring the safety and affordability of these treatments. Moreover, our focus has primarily been on addressing simpler genetic diseases. Take sickle cell anemia, for instance, which is often caused by a single mutation, making it amendable to CRISPR-based correction. However, many diseases arise from more complex scenarios involving widespread mutations, multiple genetic alterations, or involvement of multiple genes. Looking ahead, gene therapy holds the promise to expand beyond single mutations.
On the other hand, cell therapy presents a distinct approach. For instance, in treating leukemia, certain chemotherapy drugs may not completely eradicate tumor cells. Over the past two decades, researchers have discovered that by harnessing the patient's T cells, which are essential for fighting infections, these cells can be engineered to better target and eliminate tumor cells. When these modified T cells are reintroduced into the patient's body, they can effectively combat the cancer. However, cell therapies pose unique challenges due to the complexity of cellular behavior. Sometimes, injected cells can become uncontrollable, leading to unintended harm to healthy tissues, or they may be rendered ineffective due to suppression by tumor cells. CRISPR technology presents a potent tool for enhancing the precision and safety of these immune cells, ensuring they function optimally for maximum clinical benefit.
What are the other uses of CRISPR and What is its future?
Any parent will tell you how hard it can be to persuade children to eat green vegetables and salads. But CRISPR is coming to the rescue, making healthy foods taste better by dialing down the bitterness in many vegetables and enhancing the flavour of fruit.
Scientists have already used CRISPR to produce virus-, bacterial- and fungal-resistant crops that can cope with extremes of heat and cold. They’ve also increased the size of rice, wheat and maize grains and produced bigger and better soybeans and brassicas.
Professor David Savage of the University of California believes his team are close to developing varieties of rice and sorghum that will not just survive the climate crisis but actively help tackle it by capturing more carbon from the atmosphere and storing it in their roots.
Scientists from the University of Berkeley and Innovative Genomics Institute have created disease-resistant cacao plants.
Researchers at the Pasteur Institute in Montevideo, Uruguay, have used CRISPR techniques to modify the genes of farm animals to make them more disease-resistant. In one experiment, pigs were rendered immune to respiratory diseases like swine flu.
They also focused on avoiding painful procedures such as the removal of cows’ horns which is done to avoid them harming one another. The scientists introduced a gene mutation found in horn-free Angus cattle to create a hornless breed of Holstein cows.
Additionally, there are prospects in manufacturing by leveraging organisms such as yeast and bacteria to produce desired products. Consider the possibility of employing CRISPR to design novel microbes capable of significantly enhancing production, such as increasing beer output by tenfold. Furthermore, this technology could enable the creation of beer with superior taste profiles, customized to cater to diverse preferences and requirements.
Lastly, ecological engineering presents intriguing possibilities. For instance, there are efforts to eradicate specific invasive or disease-carrying mosquito species utilizing CRISPR technology. On another front, some researchers are exploring the prospect of resurrecting extinct species. Notably, there have been recent announcements regarding endeavors to revive a woolly mammoth adapted to thrive in the Arctic's frigid conditions.
What are some ethical concerns related to CRISPR?
One of the most contentious ethical issues is the use of CRISPR for editing the human germline—the genetic material passed on to future generations. Germline editing raises concerns about the potential unintended consequences and long-term effects of altering the human gene pool. There are fears of unforeseen genetic mutations, unintended off-target effects, and the potential for creating "designer babies" with enhanced traits, which could exacerbate existing social inequalities and discrimination.
According to bioengineer Stanley Qi, "Another concern is in the division of treatment, which has three categories: cure, prevention, and enhancement. Curing someone’s disease is great. Prevention, which means someone is at risk of developing a problem, is a gray area. If someone has a high chance of getting an infectious disease, should we use gene therapy to permanently modify their DNA to reduce their risk? That question really depends on if we have other options. The last category – enhancement – is likely unethical. People talk about the possibility of targeting a gene to grow more muscle or make people smarter or better looking. But if research goes into this category, only some people may be able to afford it. This could amplify the imbalance of socioeconomic status. Another facet to consider is medical necessity. Is the therapy really necessary, or are there other ways to solve the problem through currently available drugs, diet, exercise, etc.?
Beyond medicine, some scientists may want to use CRISPR for ecological reasons, for example, eliminating mosquitoes. From my viewpoint, that’s controversial because I think every species exists for a reason. If we try to eliminate mosquitoes, we might have a chain reaction that affects other life forms in the environment and can be irreversible. I hope in the future we can make this technology reversible like installing a switch so that if we make something that turns out to be less than ideal, we still have some way to reset it."
CRISPR technology has the potential for both beneficial and harmful applications. There are concerns about the misuse of gene editing tools for purposes such as bioengineering pathogens, creating bioweapons, or conducting controversial genetic experiments.
In conclusion, CRISPR technology represents a groundbreaking advancement with transformative potential across various fields, from medicine and agriculture to biotechnology and beyond. Its unparalleled precision, versatility, and efficiency have opened up new frontiers in genetic research and therapy, offering hope for addressing previously incurable diseases and improving human health. However, alongside its remarkable promise come significant ethical considerations and challenges that must be carefully navigated to ensure responsible and equitable use. By fostering collaboration, dialogue, and informed decision-making among scientists, policymakers, ethicists, and the public, we can harness the power of CRISPR for the benefit of society while upholding ethical principles and safeguarding human welfare. As CRISPR continues to evolve, it holds the potential to revolutionize our understanding of genetics and reshape the future of healthcare and biotechnology.


Comments
Post a Comment